Accutase™
Non-mammalian and non-bacterial based / Cell detachment solution
Accutase™ is a ready to use non-mammalian, non-bacterial replacement for all applications of trypsin. It is a natural enzyme mixture with proteolytic and collangenolitic activities. It mimics the action of trypsin and collagenase simultaneously. However, because the enzymes operate more efficiently than trypsin and collagenase, it results in gentler and more effective cell detachment.
Advantage of Accutase™
- Gentle and efficient dissociation of any adherent cell line
- No mammalian or bacterial components are contained
- No neutralization steps by serum or trypsin inhibitors are required
- Works extremely well on embryonic and neuronal stem cells
>> Click here to Accumax™ Cell Dissociation Solution
Cell lines tested
A few cell lines that Accutase™ has been shown to detach without harm:
| hESCs | fibroblasts |
| keratinocytes | vascular endothelial cells |
| vascular smooth muscle cells | hepatocytes |
| hepatocyte progenitors | primary chick embryo neuronal cells |
| bone marrow stem cells | adherent CHO cells |
| adherent BHK cells | macrophages |
| 293 cells | L929 cells |
| immortalized mouse | testicular germ cells |
| 3T3 | Vero |
| COS | HeLa |
| NT2 | MG63 |
| M24 and A375 metastatic melanoma | gliomas U251 and D54 |
| HT1080 fibrosarcoma cells | Sf9 insect cells |
Applications
Accutase performs exceptionally well in detaching cells for:
| hESC culturing | virus growth assay |
| analysis of cell surface markers | cell proliferation |
| apoptosis | cell haptotaxsis |
| tumor cell migration assays | routine cell passage |
| flow cytometry | quiescence assays by serum starvation |
| transformation assays by oncogene | transfectionneural crest cell migration assays |
| production scale-up (bioreactor) |
Cell Detachment Result
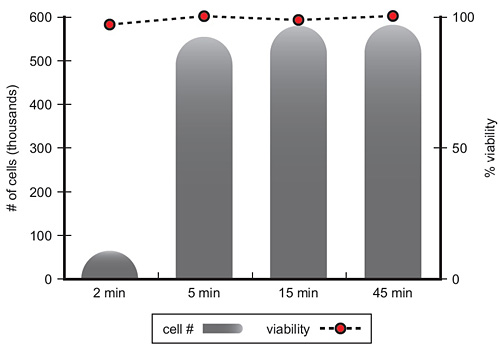
Typical cell passaging protocol using Accutase™
Accutase is formulated at a concentration that is ready to use, once defrosted. (Note: Never defrost a bottle of Accutase at 37°C.) A defrosted bottle of Accutase can be removed from the refrigerator and immediately applied to cells. It does not need to be and should not be pre-warmed to 37°C. Accutase contains proteolytic and collagenolytic enzymes to gently break down the cell adhesion structure on the outside of cells that attaches them to the bottom of the flask.
This entire procedure should be done in a laminar flow hood using proper aseptic technique.
- Carefully aspirate all of the media from the cell culture flask. (Rinsing with PBS is not necessary.)
- Immediately add enough Accutase to the flask to cover the cells. (Typically 2.5 to 5ml for a T25 flask depending upon confluency and density of the cell culture.)
- Set the flask aside at room temperature (RT) for 5 to 10 minutes up to a maximum of 1 hr. Check the flask frequently to see if the cells have rounded rather than merely shrunken or no longer appear "spidery" while remaining attached to the bottom of the flask.
- Once the cells have turned into "balls", smack the flask against the palm of your hand to dislodge any "stickers".
- Gently disperse the cells and take a sample of the cell suspension to determine the viable cell density.
- Add an aliquot of the detached cells to fresh media in new flasks. Place the flasks into the 37°C incubator. No neutralization steps are required. The cells will reattach within a few minutes depending upon cell type.
Passaging Blood or Bone Marrow Derived Macrophages Using Accutase™ Cell Detachment Solution
Primary macrophage cultures may be derived from density gradient separated whole blood or bone marrow by culturing these isolates in the presence of specific growth factors in order to encourage the growth, expansion and differentiation of the macrophage cell type. A literature search will yield several in-depth protocols which out-line these isolation techniques in detail. Once harvested and put into culture at 37oC in a 5% CO2 incubator the macrophage progenitor cells will adhere to tissue culture flasks and will not be washed away when the media is changed in order to remove any non-adherent cells and allow for macrophage differentiation/maturation.
To remove the Primary macrophage cultured cells from the tissue culture plates for further analysis:
- Remove the supernatant from the flask (T25) or tissue culture dish (15mm).
- Wash the plate or dish with 5ml sterile PBS and remove it.
- Add 5ml of Accutase™ to the flask or dish and incubate at room temperature for 10-15 minutes. Inspect the flask under the inverted microscope to look for cell “shrinkage” or detachment.
- Gently swirl the flask and add another 5ml of Accutase™. Incubate the cells another 10-15 minutes at room temperature. Re-suspend the cells by trituration (pipetting up and down several times) being careful not to cause bubbles.
- Count the cells and adjust the concentration to be used for further analysis.
NOTE: This procedure may also be carried out on ice which may decrease the incubation times needed.
Passaging Macrophage Cell Lines such as DH82 and RAW264.7
Using Accutase™ Cell Detachment Solution
For those investigators who prefer to utilize immortalized cell lines rather than isolate primary cultures, Accutase™ may be used to passage their macrophage lines in a similar manner to the techniques used for most other adherent cell lines.
- Remove flask (T25) from the incubator and examine it under the inverted microscope to check for cell confluency.
- Transfer the flask to the tissue culture hood for cell passaging. Remove the media from the flask. Wash the flask 2 times with 5ml of sterile PBS; fully remove after each addition.
- Add 5ml of Accutase™ to the flask and incubate it at room temperature for 5-10 minutes.
- Inspect under the microscope for classic signs of cell detachment ie, “shrinkage” or “rounding”.
a. If there is significant signs of “rounding”, then transfer the plate back to the hood and triturate (pipette up and down several times) being careful to not create bubbles.
b. If cells do not appear significantly “rounded” then allow them to incubate and additional 2-3minutes and proceed as in step “a” above. - Passage cells at a 1:2 or 1:4 ratio every 3-6 days.
Please note that different adherent cells stick to tissue culture plastic with varying degrees of adherence. For this reason the incubation time required for detachment can vary with some cell types needing more or less time to detach. In the case of extremely tenaciously adherent cells our stronger formulation, Accumax may work better.
Dissociation of NSCs and Neurospheres with Accutase™
Dissociation of adherent human or rat NSCs
- Aspirate the medium from culture dish.
- Add 2 mL of Accutase™ to culture dish.
- Incubate for 2 to 5 minutes at 37 °C until individual single cells start to round up.
- Gently rinse to remove cells off of the plate’s surface.
- Transfer cell suspension to 15 mL conical tube. Gently pipette up and down until cells are in a single cell suspension.
- Add 8 mL of medium to rinse any remaining cells off of the dish’s surface and transfer to the conical tube (from Step 5).
- Take a 20uL sample of the cell suspension to determine viable cell density.
- Centrifuge conical tube containing the cell suspension at 200g for 4 minutes.
- Aspirate supernatant, resuspend in fresh medium and plate on coated dish(s). Incubate at 36 to 38°C in a humidified atmosphere of 4 to 6% CO2 in air.
Dissociation of human or rat neurosphere cultures
- Remove neurosphere cell suspension from culture dish and transfer to a 15 mL conical tube.
- Let neurospheres settle down in the tube (~2 to 5 minutes) before proceeding to Step 3. Alternatively, the cells can be centrifuged at 100g for 1 minute.
- Gently aspirate medium leaving the neurospheres at the bottom of tube with approximately 100 μL of media remaining.
- Resuspend neurospheres in 5 mL DPBS.
- Let neurospheres settle down in the tube (~2 to 5 minutes) before proceeding to Step 6. Alternatively, the cells can be centrifuged at 100g for 1 minute.
- Gently aspirate DPBS leaving the neurospheres at the bottom of tube with approximately 100 μL of DPBS remaining.
- Add 1mL of Accutase™ to the neurospheres and incubate 10 minutes at room temperature.
- Using the proper sized pipette tip (i.e.1000 μl), pipette up and down until all the neurospheres are in a single cell suspension.
- Add 4mL of fresh medium to the tube.
- Centrifuge the cells at 200g for 4 minutes.
- Gently aspirate the supernatant.
- Resuspend cells in fresh medium, transfer to a new culture dish and incubate at 36 to 38°C in a humidified atmosphere of 4 to 6% CO2 in air.
Q&A
A: Accutase can be used whenever gentle and efficient dissociation of any adherent cell line is needed. Accutase is a direct replacement for trypsin.
Q: What is the difference between Accutase and Accumax?
A: Accumax contains the same proteolytic and collagenolytic enzymes as Accutase, but is formulated at a concentration that is 3X higher and does not contain phenol red.
Q: What color should my Accutase be when it arrives?
A: Please see this document for information, including photos, about the effects of shipping on the color of Accutase - what's normal and what's not.
Q: Can I use Accutase to dissociate non-adherent clumpy cells?
A: Yes. Accutase can be used to dissociate spheres of neural progenitors (neurospheres). However, if the clumps do not dissociate completely, then consider using Accumax which contains the Accutase enzymes, but at a higher concentration.
Q: The frozen bottle of Accutase shows uneven color distribution and upon thawing it has layered color distribution. Is this normal?
A: Yes. During the defrosting procedure or shipping, uneven color can be observed. However, this will not compromise the activity or performance of Accutase. Just be sure to mix the defrosted Accutase by inverting the bottle to get even color before use.
Q: The Accutase or Accumax arrived partially thawed. Can I still use it?
A: Yes. As long as the product contains an "ice-cube" or is cool to the touch it can be placed in the refrigerator for later use. It also can be refrozen for long term storage (if frozen within two months of receipt).
A: If it was exposed to 37 °C just until complete thawing was achieved without raising its temperature to 37 °C, it still can be used. However, it could result in decreased enzyme activity and it may take more time to get full dissociation. If you observe this, consider starting with a new bottle. If a bottle of Accutase or Accumax is kept at 37 °C for more than one hour, it will lose its activity.
Q: Do I need to aliquot and refreeze Accutase or Accumax after I defrost a bottle?
A: No. Once thawed, it is stable for at least 2 months in the refrigerator if stored promptly after use.
Q: Do I need to wash my cells before adding Accutase?
A: No - pour off the media and add enough Accutase to cover your cells and wait for them to "ball up".
Q: Do I need to stop the dissociation action of Accutase with serum?
A: Usually not. Accutase is gentle enough that only dilution of the reagent with DPBS or media is required to stop the dissociation activity. In the unusual cases where inactivation is required, the standard trypsin inhibitors will work, such as soybean trypsin inhibitor.
Q: Do I need to wash Accutase out after using?
A: No, this is one of the advantages of using Accutase.
Q: Do I need to dilute Accutase before use?
A: No. Accutase is supplied as a convenient, ready to use reagent. No dilution is required prior to use.
Q: How long should I leave the Accutase on my cells?
A: Detachment times for Accutase are similar to trypsin. This time should be determined empirically. Look under the microscope and watch them turn from spidery to little balls. In general, cells can be left in Accutase without damage for a much longer time than trypsin.
Q: What is Accutase made from?
A: Accutase contains no mammalian or bacterial components. It is a natural enzyme mixture with proteolytic and collagenolytic enzyme activity. This means it mimics the action of trypsin and collagenase at the same time. However, because it is more efficient than mammalian trypsin & collagenase, it is formulated at a much lower concentration making it less toxic, and gentler but just as effective.
Q: Do I need to worry about over-dissociating my cells with Accutase?
A: No. However, although Accutase is gentle on cells, the optimal time for dissociation should be determined for your specific cell type and application.
Reference
- Yotsumoto, Karen, et al. "Amelogenin Downregulates Interferon Gamma-Induced Major Histocompatibility Complex Class II Expression Through Suppression of Euchromatin Formation in the Class II Transactivator Promoter IV Region in Macrophages." Frontiers in Immunology 11 (2020): 709.
- Inamori, Sachiko, et al. "Modeling early stages of endoderm development in epiblast stem cell aggregates with supply of extracellular matrices." Development, Growth & Differentiation (2020).
- Deng, Xiaoyue, et al. "Characterization of human induced pluripotent stem cells carrying homozygous RB1 gene deletion." Genes to Cells (2020).
- Kikuchi, Tetsutaro, and Tatsuya Shimizu. "Thickness-wise growth technique for human articular chondrocytes to fabricate three-dimensional cartilage grafts." Regenerative therapy 14 (2020): 119-127.
- Tozaki-Saitoh, Hidetoshi, et al. "Involvement of exchange protein directly activated by cAMP and tumor progression locus 2 in IL-1β production in microglial cells following activation of β-adrenergic receptors." Journal of Pharmacological Sciences (2020).
- Kusumoto, Junya, et al. "OPN4 belongs to the photosensitive system of the human skin." Genes to Cells 25.3 (2020): 215-225.
- Shigeto, Kawai, et al. "Three-dimensional culture models mimic colon cancer heterogeneity induced by different microenvironments." Scientific Reports (Nature Publisher Group) 10.1 (2020).
Downloads
Ordering Information
| Product | Cat.No. | Storage | PKG Size | Price(US$) | |
|---|---|---|---|---|---|
| Accutase™ | NU1267954 | -20°C | 100 ml | 29.00 | Buy |
