About Sunniest
Developed a new bonded phase made with unique end capping!
|
This special C18 reagent consists of C18 chain part and endcapping part like an arm. An arm of this reagent moves like a Geometrid caterpillar, so that a functional group on the tip of the arm can bond with a silanol group which is located anywhere. After bonding this unique C18 reagent an end-capping is densely done with trimethysilane (TMS) using SAC technique.(1)
(1)SAC technique is developed by Chromanik Technologies Inc. for Sunrise C18-SAC is an operation technique that silanol groups are converted into asiloxane bond under high temperature conditions. |
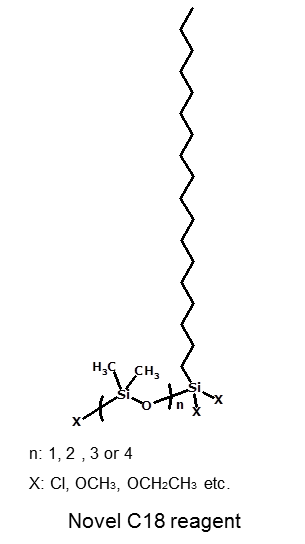 |
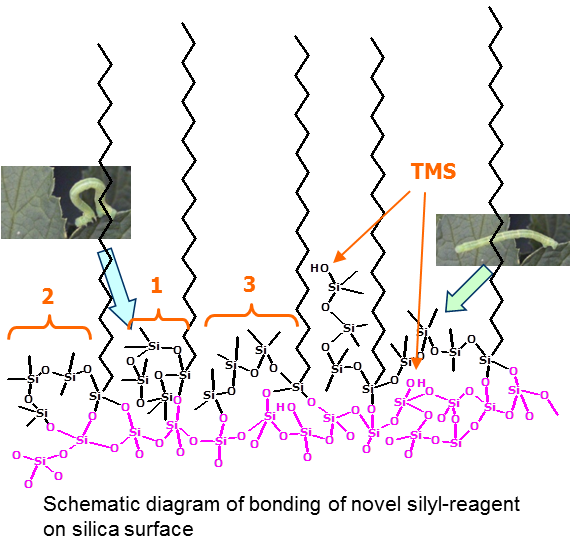 |
Evaluation of the end-capping
 |
|
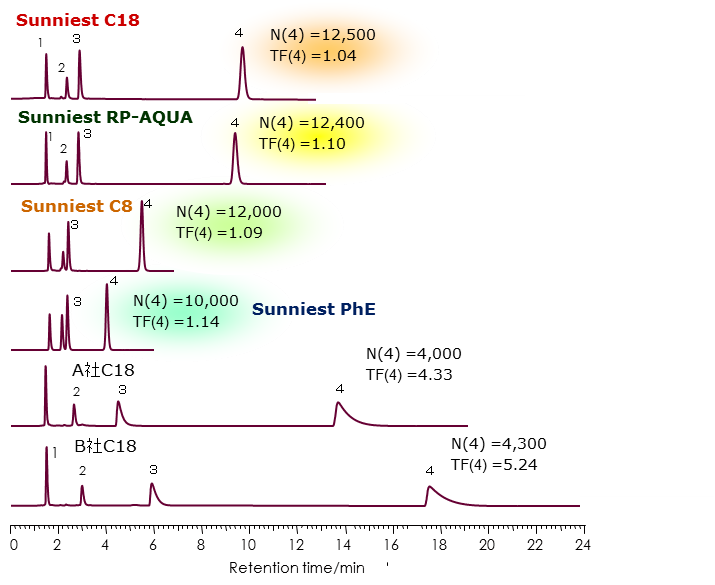
Amitriptyline peak becomes a tailing peak by influence of residual silanol groups and many HPLC manufacturers use amitriptyline as an index of the amount of silanol groups. We evaluated the amount of residual using amitriptyline.
It is generally known that a peak tailing for a basic compound occurs more using acetonitrile in the mobile phase than using methanol although methanol has been used as an organic solvent in the mobile phase for separating basic compounds.
Sunniest C18, RP-AQUA, C8 showed sharp peaks without tailing even with acetonitrile. In other words, the Sunniest C18, RP-AQUA, C8 column, without the use of organic solvent is limited, can be set to a wide range of mobile phase conditions, you can construct an optimum separation conditions for all of the components.
Lineup
| Particle | USP L No. | Particle size (µm) | Bonding | Characteristic |
|---|---|---|---|---|
| C18 | L1 | 1.8, 3, 5 | C18 | First Choice |
| C18-HT | L1 | 2 | C18 | |
| RP-AQUA | L62 | 3, 5 | C28 | 100% aqueous mobile phase is available |
| C8 | L7 | 3, 5 | C8 | For high-speed analysis of low-polar compounds |
| PhE | L11 | 3, 5 | Phenylethyl | Analysis of separation of aromatic compounds |
| PFP | L43 | 5 | Pentafluorophenyl | Analysis for structural isomers and halide |
