iMatrix-511(recombinant laminin-511)
Cell culture substrate / Recombinant human Laminin511-E8 fragment
 |
|

“iMatrix-511” and “iMatrix-511 silk” are recombinant human laminin-511 E8 fragment for cell culture. Below are the features possible of laminin-511 E8 fragment on ES/iPS cells and mesenchymal stem cells (MSC).
Features:
- Use of feeder-free culture medium
- Enhanced cell attachment
- Rapid recovery of cell-cell contacts
- Simple single cell passaging
- Optimum cell yield and viability
ECM Pre-mix method (WITHOUT PRE-COATING)
Skip the time-consuming process
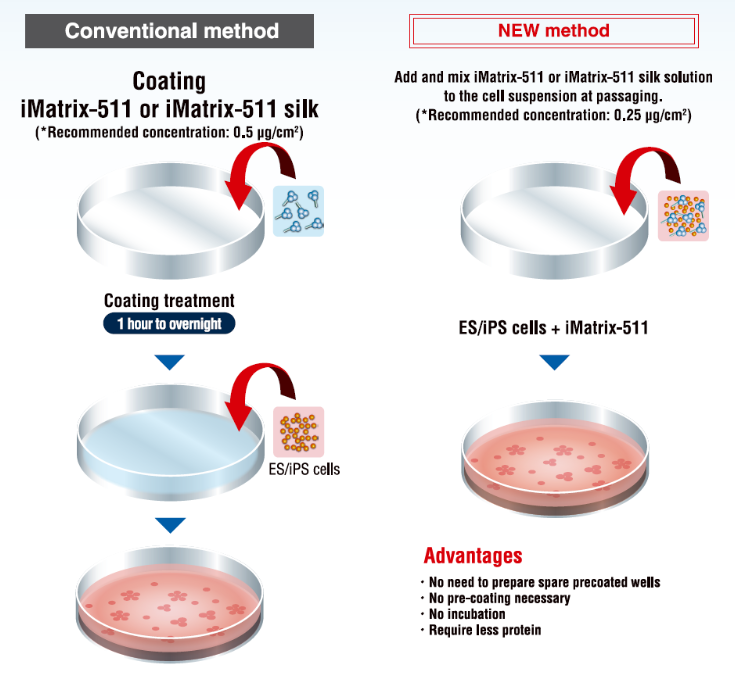
Reference:
Miyazaki, T., Isobe, T., Nakatsuji, N., & Suemori, H. . Efficient Adhesion Culture of Human Pluripotent Stem Cells Using Laminin Fragments in an Uncoated Manner. Scientific Reports, 7(41165), 1-8, (2017)
Laminin-511E8 fragment
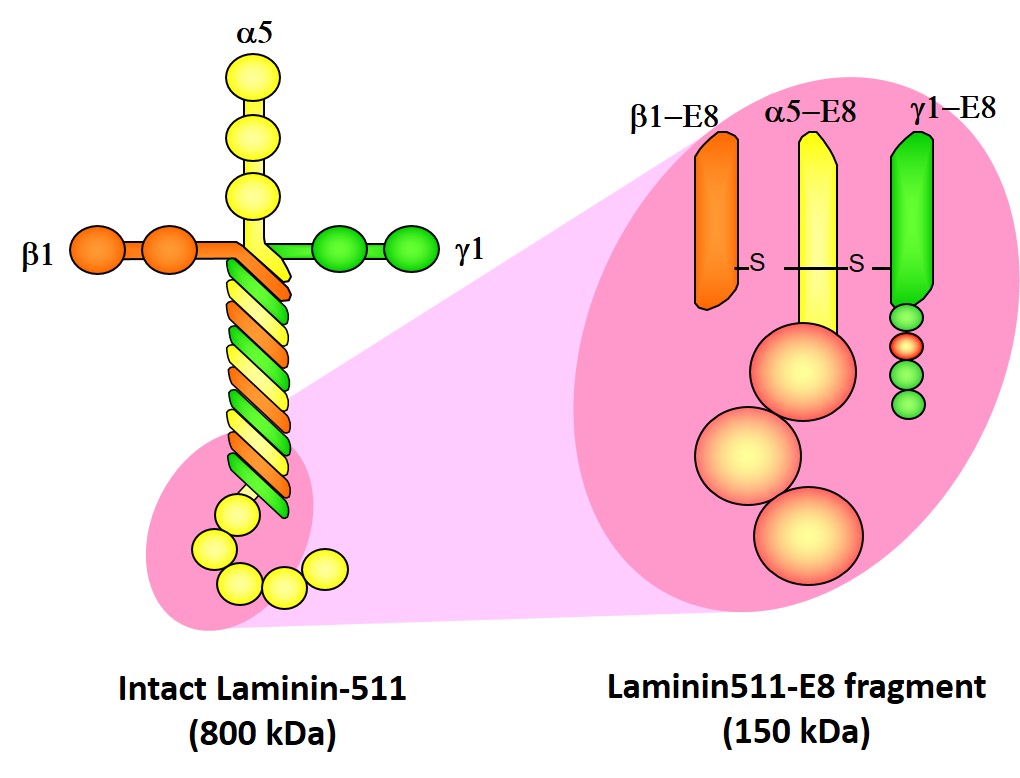
Laminin-511 is a major component of basement membrane that can function as a scaffold for pluripotent stem cells (ES/iPS cells). As a part of the basement membrane, laminin-511 (800 kDa) binds to integrins on cell surfaces. Laminin-511 is a very large protein (800 kDa) composed of three chains (α-, β-, and γ- chains), and forms supramolecular aggregates. It is difficult to produce as a recombinant protein. As we fragmented Laminin-511 and searched for the smallest component that binds to integrin on the cell surface, we identified the component called Laminin-511 E8 fragment (150 kDa). We decided to produce this component as a recombinant protein.
Human iPS cells adhere more strongly to Laminin-511E8 fragment than to the “Laminin-511 (Intact).”
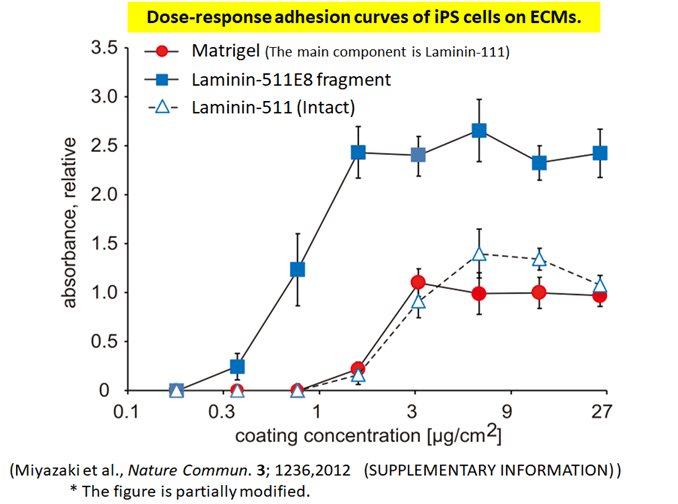
Human iPS cells adhere more strongly to Laminin-511 E8 fragment than to the "Laminin-511 (Intact)".
Laminin-511 E8 fragment and other substrates were compared for their cell attachment.
The absorbance (OD570) represents the relative number of attached cells normalized against the values at the maximum effect on Matrigel, which was arbitrarily set as 1.
This result confirmed that Laminin-511 E8 fragment adhere to cells most strongly.
Forming highly dense colonies
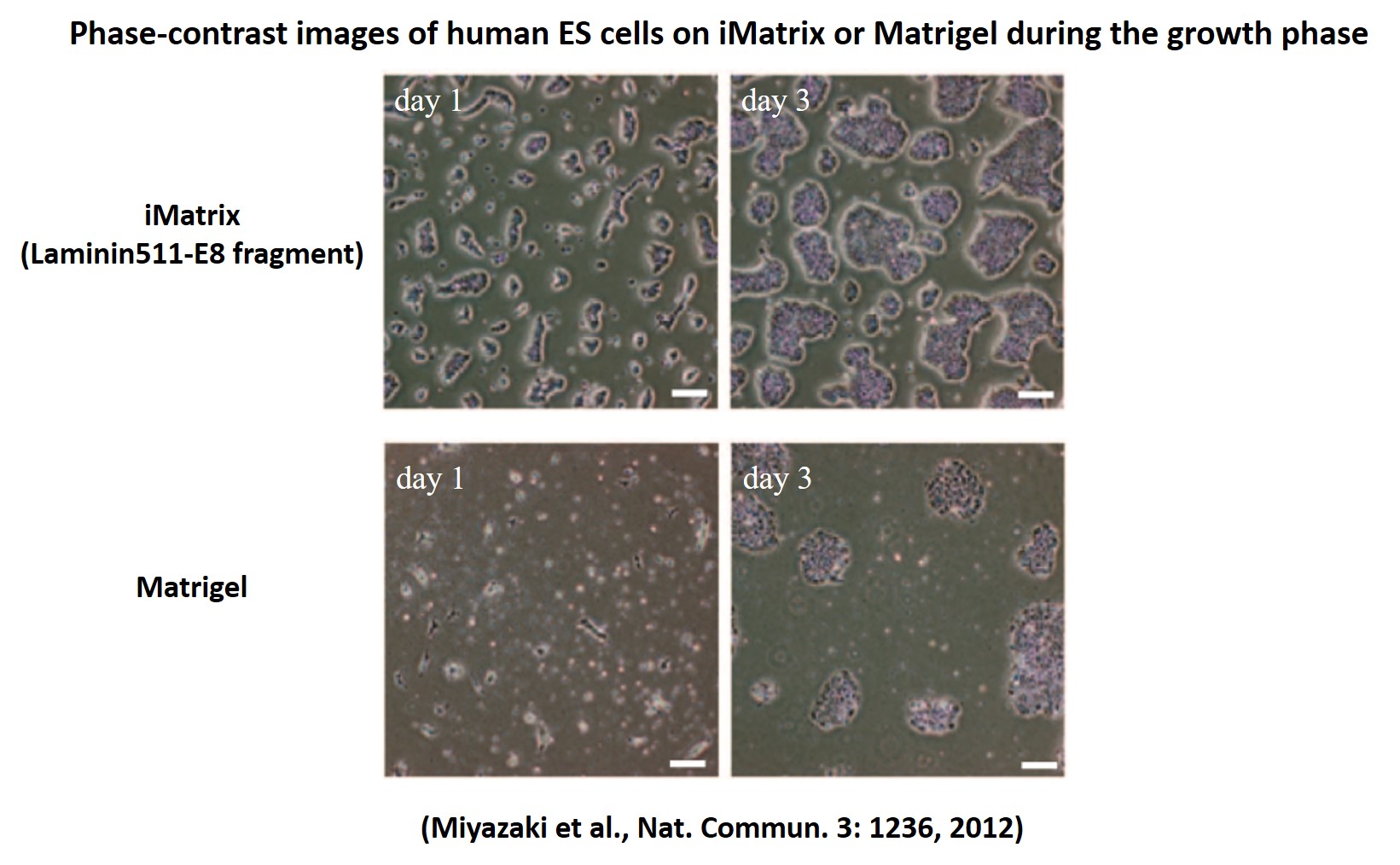
Dispersed human single ES cells adhere rapidly to Laminin-511 E8 fragment and proliferate immediately. We passaged human ES cells on Laminin-511 E8 fragment and other cell culture substrates. Single human ES cell colonies at the time of passage confirmed that Laminin-511 E8 fragment adhere rapidly to cells and proliferate.
Further advantages of using Laminin-511E8 fragment in ES/iPS cell culture
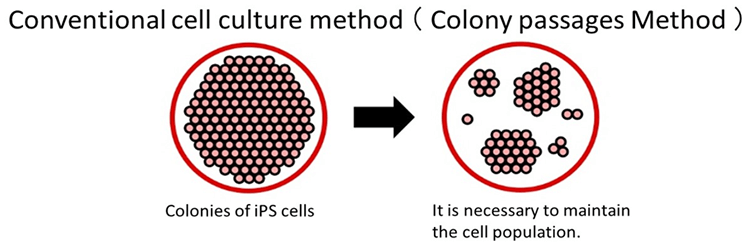
With conventional culture methods, cells die if ES/iPS cells are dispersed into single cells.
Therefore, maintaining a cell colony is necessary to a certain extent when passaging.
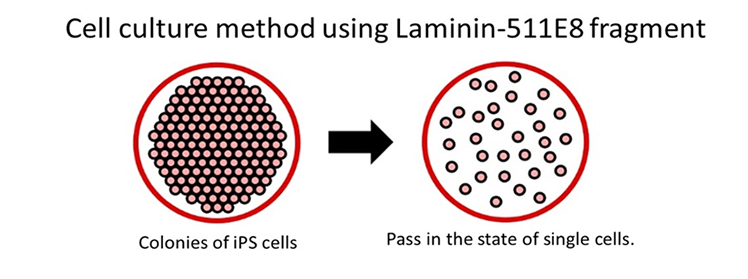
Using Laminin-511 E8 fragment as cell culture substrate makes it possible to maintain and culture even a single ES/iPS.
-> The period of time required for learning cell culture methods is reduced.
-> The extension efficiency of cell culture is drastically enhanced.
Growth of ES/iPS cells using Laminin-511E8 fragment
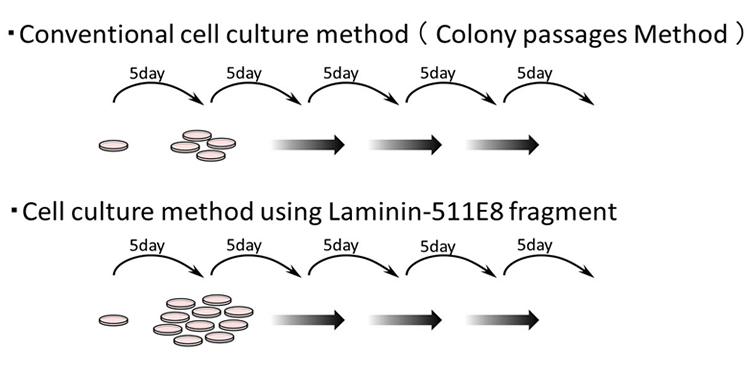
*5 days
*colony passaging method
While the extension efficiency was 1:4 at the time of passage with the conventional culture method (colony passage), the use of Laminin-511 E8 fragment as cell culture substrate has enabled extension to be 1:100.
Volume production of ES/iPS cell culture using Laminin-511E8 fragment
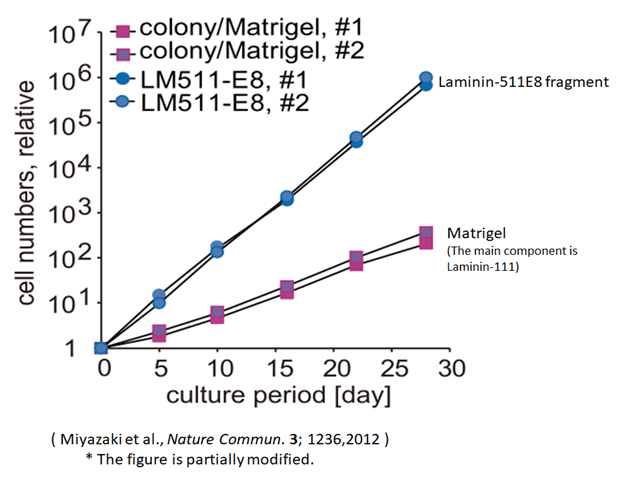
We compared the numbers of ES/iPS cells cultured by the conventional method (colony method) for 30 days and those cultured by using Laminin-511 E8 fragment as cell culture substrate. The horizontal axis of the graph shows the number of culture days, and the vertical axis, the number of cells.
The results confirmed that the number of cells multiplied by 200 or more with the use of Laminin-511 E8 fragment in comparison with the conventional method (colony method).
Publication
Matsuoka Lab @Northwestern University: Hsiang-Tsun Chang et al. An engineered three-dimensional stem cell niche in the inner ear by applying a nanofibrillar cellulose hydrogel with a sustained-release neurotrophic factor delivery system. Acta Biomater. 108: 111, 2020.
Nakauchi Lab @Stanford University: Nishimura T. et al. Sufficiency for inducible Caspase-9 safety switch in human pluripotent stem cells and disease cells. Gene Ther. 2020.
Matsuoka Lab @Northwestern University: Rachel A Heuer et al. Three-Dimensional Otic Neuronal Progenitor Spheroids Derived from Human Embryonic Stem Cells. Tissue Eng Part A. Online ahead of print, 2020.
Kaneko Lab @CiRA, Kyoto University: Iriguchi S et al. A clinically applicable and scalable method to regenerate T-cells from iPSCs for off-the-shelf T-cell immunotherapy. Nat Commun, 2021.
Sasaki Lab @University of Pennsylvania: Sakata, Y. et al. "Reconstitution of human adrenocortical specification and steroidogenesis using induced pluripotent stem cells". Dev Cell, 2022. <<NEW!
