Ceglu™ Pre-coated Plate for Easier Stem Cell Culture
Chemically-defined matrix / scaffold
Ceglu™ is fully chemically defined matrix for cell culture. It provides a consistent and uniform cell culture surface across various devices and substrates, ensuring reproducible environments from research to manufacturing.
Ceglu™ cultureware consists of cell culture plate pre-coated with Ceglu™, eliminating the need for additional coating steps. It is specifically designed to support stable and reliable cell culture of iPSCs, ESCs, MSCs, and a wide range of differentiated cells
Features
- Scalable cell culture platform from research to manufacturing
- Ready-to-use (sterilized and pre-coated)
- 1 year shelf life at room temperature
- Animal-Component Free
- Various cell culture applications (For detail, please refer to the “Application note” )
Cell Culture Performance
Ceglu™ cultureware performs equivalently to protein matrix in iPSC culture, maintaining both pluripotency and undifferentiated state
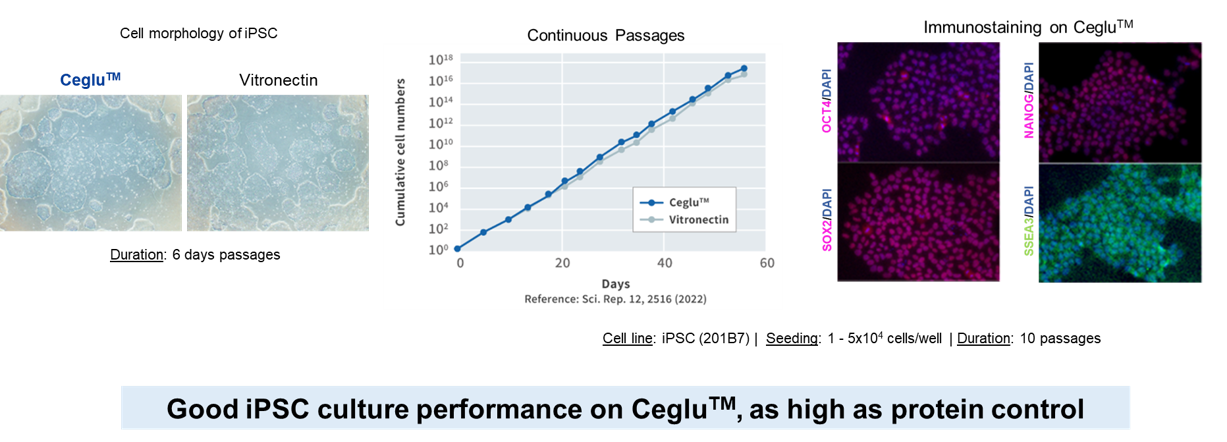
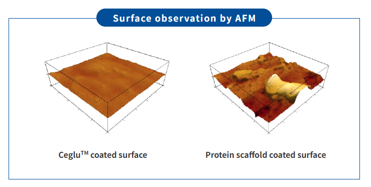
Reproducibility of cell culture through uniform surface
The uniform culture surface of Ceglu™ cultureware has been confirmed through atomic force microscopy (AFM).
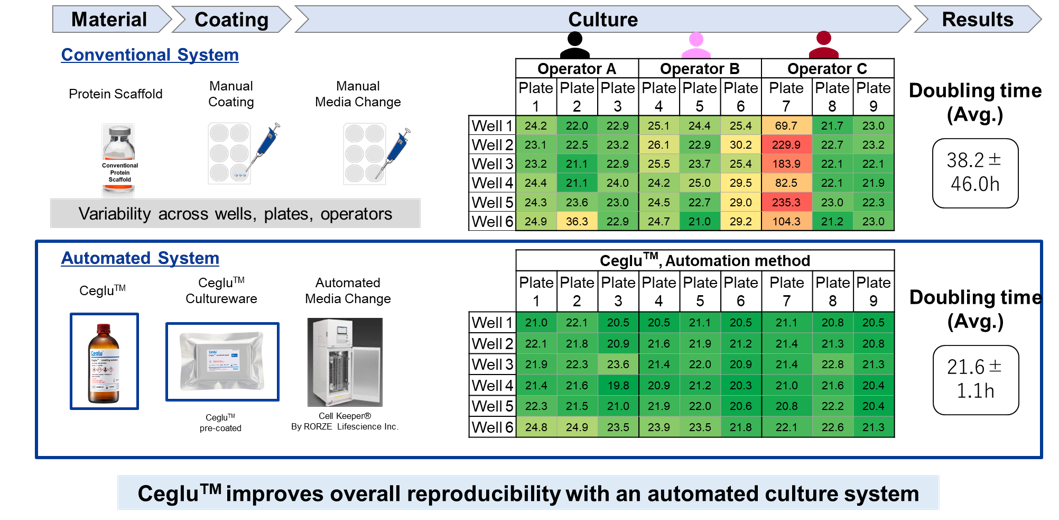
This feature enables stable cell culture with high reproducibility. The comparison below illustrates two experimental systems: one in which a protein matrix was manually coated onto plate and cultured by skilled technicians, and another using Ceglu™ cultureware pre-coated with the matrix combined with automated medium exchange. As a result, experiments integrating Ceglu™ with an automated culture system significantly reduced variability in the doubling time of iPSCs.
For details please refer to the application note “Reproducible automated iPSCs culture using Ceglu™”.
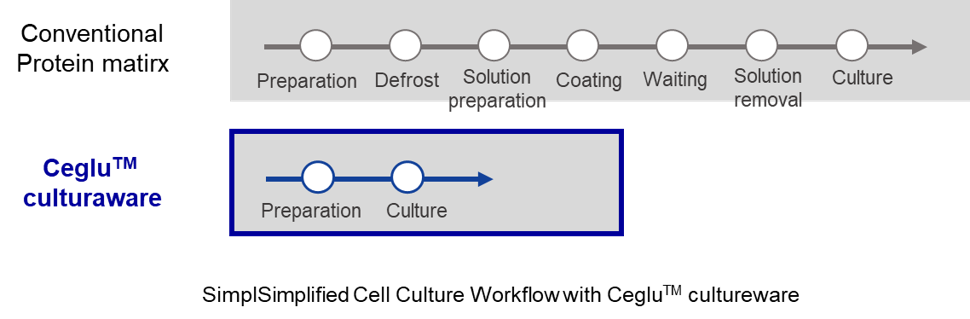
Simplified cell culture workflow
Typically, culturing iPSCs and differentiated cells requires coating culture vessels with a protein matrix. Ceglu™ eliminates this step, enabling a simplified and more efficient cell culture workflow.
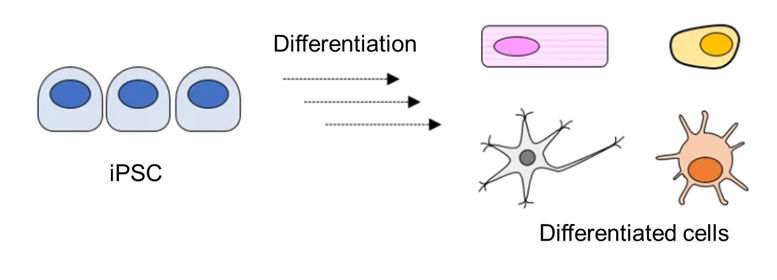
Differentiation of iPSCs
iPSCs cultured on Ceglu™ can be differentiated into a wide range of cell while maintaining a consistent, chemically defined matrix environment.
[Example] Three-germ layer, Cardiomyocytes, Hematopoietic progenitor cells, Monocytes, Hepatoblasts, Neural progenitor cells, Neurons (Dopaminergic neural progenitor cells), Glial cells (Astrocytes), etc.
For details, please refer to the differentiation application note.
Application Note (Download)
・Generation of iPSCs using Ceglu™
・Reproducible automated iPSCs culture using Ceglu™
・Differentiation of iPSCs into all three germ layers on Ceglu™
・Differentiation of iPSCs into neural progenitor cells and astrocytes using Ceglu™
・Differentiation of iPSCs into cardiomyocyte using Ceglu™
・Differentiation of iPSCs into hematopoietic progenitor cells and monocyte using Ceglu™
User guide and Product information (Download)
FAQ
| Question | Answer |
|
Are animal-origin components used? |
Ceglu™ is chemically defined materials and NOT contains animal-origin components |
|
Is there any cytotoxicity? |
Cytotoxicity and other tests was conducted with reference to the US Pharmacopeia (USP87, USP88) test methods, and NO cytotoxicity and other toxicity have been confirmed. |
|
What cell culture media can be used? |
We have extensive experiences culturing iPS cells in various media, including StemFit® AK02N, StemFit® AK03N, StemFlexTM, TeSRTM-E8TM, mTeSRTM1, Essential 8TM and others. |
|
What cell dissociation reagents can be used? |
Enzyme-based and chelating reagents can be used for cell dissociation. |
|
What should be particularly noted when using Ceglu™? |
Each data set was obtained from single passage protocol. Clumps passages is not recommended. We also recommend implementing an acclimation process when switching from other matrix. |
|
How can I culture on a large scale with Ceglu™? |
Please contact us |
Publication
A chemically-defined plastic scaffold for the xeno-free production of human pluripotent stem cells
Scientific Reports. 2022 Feb 15;12(1):2516.
https://doi.org/10.1038/s41598-022-06356-8
Adhesion Characteristics of Human Pancreatic Islets, Duct Epithelial Cells, and Acinar Cells to a Polymer Scaffold
Cell Transplantation. 2022 Sep;31:09636897221120500.
https://doi.org/10.1177/09636897221120500
Improved Production of Induced Pluripotent Stem Cells Using Dot Pattern Culture Plates
Tissue Engineering Part C: Methods. 2023 Sep 1;29(9):410-23.
https://doi.org/10.1089/ten.tec.2023.0068
Size control of induced pluripotent stem cells colonies in two-dimensional culture for differentiation into functional monocyte-like cells
Cytotherapy. 2023 Dec 1;25(12):1338-48.
https://doi.org/10.1016/j.jcyt.2023.08.002
